Technology Platform
Proprietary Electro-Optic Polymer Chemistry
What is electro-optics?
Materials are called electro-optic when they enable interactions between applied electric fields and light passing through them. Notably, they change the refractive index seen by the light with minimum loss. The result is an instantaneous and accurate conversion of an electrical signal to an optical signal. Optical signals are better for transmission over distance: an increasingly useful feature as digital signal speeds are now reaching the GHz and THz ranges and the corresponding electrical transmission distances are shrinking to meters and centimeters.
How is the polymer electro-optically active?
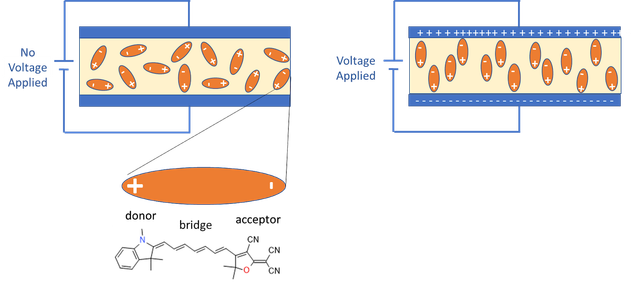
- Electro Optical, (EO), polymers are engineered materials, made by embedding a variety of specially designed electro-optic chromophore molecules into a wide range of standard host polymers
- Chromophores are “hyperpolarizable”, meaning their electron clouds are easily pulled into a different shape by the applied electric field, thereby changing their optical properties such as index of refraction.
- The combination of EO polymers and chromophores results in intrinsically superior performance in speed and sensitivity to electric field when compared traditional electro-optic materials such as Lithium Niobate, Indium Phosphide and Silicon.
- For electro-optic applications, chromophores are “poled” – or aligned -in constant directions by applying a strong electric field along with heat.
- Cooling chromophores after the molecules are in place traps them in their active state even after the poling field is removed.